In less than 20 years, autism rates have tripled to the point where one in 44 children are diagnosed with the disease. And yet, the causes of autism remain largely unknown and no treatments or preventative methods exist. David Amaral, a professor of psychiatry and director of the MIND Institute at the University of California, Davis, is one of many researchers around the globe trying to change that. As part of his research, Dr. Amaral is developing rhesus monkey models to advance studies of two forms of autism.
Autism spectrum disorder encompasses a range of disabilities, often emerging when individuals are as young as twelve months old. From the best player on the softball team or the most diligent high-school teacher, anyone could be on the spectrum. Autism can be mild enough to allow someone to carry out their day-to-day activities or so debilitating that it prevents them from functioning independently. Given the extreme differences in the severity of the spectrum, understanding and treating autism is an immense challenge.
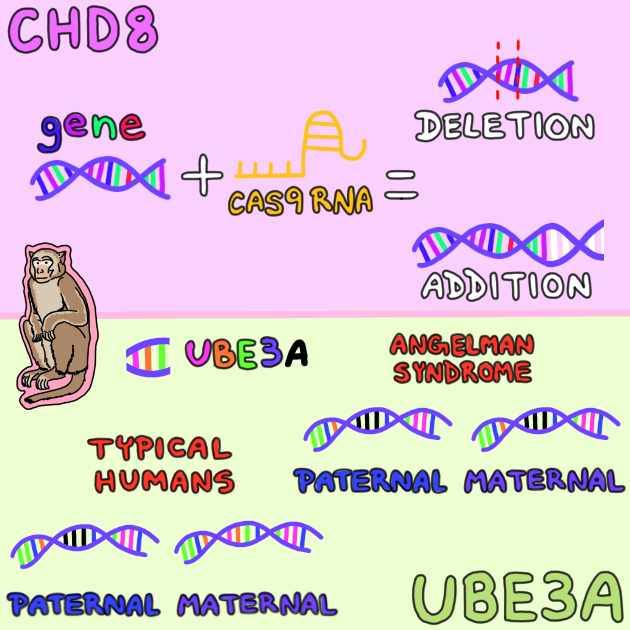
Amaral’s lines of inquiry explore the most severe forms of autism, each associated with a different gene: CHD8 and UBE3A.
Studying how to correct defects and mutations once they’re detected, Amaral’s research could lead to targeted treatments to reduce or even eliminate problems associated with autism, such as anxiety, sleep disorders and seizures. But before these advances can be studied in people, they must be validated in animals first. Amaral focuses his studies on autism in non-human primates (NHPs), primarily the rhesus monkey.
A troublesome mutation: CHD8
In his research, Amaral is trying to develop a rhesus monkey with a mutation of CHD8, and therefore, replicate — or model — autism in humans. Nearly 90 percent of people with this mutation will have autism, so if the findings from the monkey model can lead to the mutation being corrected, it could greatly reduce the prevalence of autism worldwide.
One of the strategies researchers are using is the genome editing technique, CRISPR/Cas9. Like an editor drags, drops and removes information in a document, tools like CRISPR/Cas9 edit and delete genes from a DNA sequence. This tool allows Amaral to modify the gene to develop the monkey model. With this technique and in vitro fertilization, Amaral is building a colony of rhesus monkeys with CHD8 mutations.
“[With our models] we can use advanced imaging techniques like MRI, observe their behavior and look at their brains to see what’s different,” says Amaral.
Turning on the light of Angelman Syndrome: UBE3A
The second form of autism Amaral’s team is studying is Angelman syndrome, associated with the gene UBE3A. Among the severest forms of autism, Angelman leads to smaller brains, seizures, and speech and balance problems.
Amaral describes his approach to investigating Angelman as similar to turning on a light. In all people, the UBE3A’s paternal gene is turned off, and the maternal gene is lit up and producing protein. However, in people with Angelman syndrome, both the maternal and paternal genes are dark, so neither gene produces proteins needed to avoid the autistic characteristics of the syndrome. Amaral’s theory is that by lighting up the maternal or paternal gene, the debilitating effects of the syndrome could be reduced.
Before testing these ideas in the rhesus monkey model, Amaral has been turning on the genes in mouse models. The results of the experiments have been promising so far.
“When you do that, the mouse’s condition improves,” Amaral reports.
Looking ahead to monkey models and the hope of improving lives
Having successfully experimented on mice, Amaral’s monkey models will be the next site of his research.
Amaral hopes for a future where someone could check before birth for a mutation in the UBE3A gene, which would predict if the fetus will have Angelman syndrome. Using this genetic engineering technique to light up the UBE3A’s maternal or paternal gene could allow people with the mutation to give birth to a healthy baby. Before scientists take this technique to people however, it needs to be brought to NHP models, like those being developed by Amaral. Mouse models are simply a stepping stone.
The global focus on autism research may allow doctors to predict, treat and prevent autism. Amaral expects it will take three to five years to develop the NHP models that can then be used to develop potential therapies for humans.
“With nonhuman primate models, we could be on the verge of a huge revolution,” he says.
- Autism spectrum disorder encompasses a range of disabilities, often emerging when individuals are as young as twelve months old.
- The causes of autism remain largely unknown and no treatments or preventative methods currently exist.
- One of the strategies that researchers are using is the genome editing technique CRISPR/Cas9. This tool allows scientists to modify a key gene involved in autism in order to develop a monkey model of the disease.
Sources
David Amaral, et al. “The Amygdala and Autism: Implications from Non-human Primate Studies.” National Library of Medicine, October 2003. 10.1034/j.1601-183x.2003.00043.x.
“Gene Therapy Shows Early Promise as Angelman Syndrome Treatment.” University of North Carolina Health and University of North Carolina School of Medicine. October 22, 20221. https://news.unchealthcare.org/2021/10/gene-therapy-shows-early-promise-as-angelman-syndrome-treatment/
Interview with Dr. David Amaral. Interview by Tara Prakash. July 25, 2022
Interview with Dr. David Amaral. Interview by Robert MacNeil. April 19, 2011
“New Models: Gene-Editing Boom Means Changing Landscape for Primate Work.” Nature Medicine. November 8, 2016. https://www.nature.com/articles/nm1116-1200
Qiong Xu, et al. “Autism-associated CHD8 deficiency impairs axon development and migration of cortical neurons.” BioMed Central, December 19, 2018. https://doi.org/10.1186/s13229-018-0244-2
Bollard, Gavin. Life with Aspergers. Blogger. October, 2007.
Johnson, Malcolm. Asperger Management. Blogger. 2020.
Editorial Team
- Chief Editor: Juhi Amin
- Team Editor: Raunak Banerjee
- Creative Team Senior Managers: Daniela Benoit, Bebe Lemanowicz
- Creative Team Manager: Annika Singh
- Social Media Team Managers: Spencer Lyudovyk, Yoojin Jeong
- Image Credits: Daniela Benoit
Mentor
Casey Williamson is a science writer and communications strategist who has covered human and animal health and research at Michigan State University and Oregon Health & Science University and the University of Texas. Prior to entering the world of science writing, she was a communications manager in the tech industry in Austin, Texas. Over the course of 20 years, she has mentored high school and college students and incited young scientists to communicate passionately and clearly about their work.
Content Expert
David Amaral, Ph.D., is a professor of psychiatry at the University of California, Davis, and is the founding Research Director of the MIND Institute. His expertise is in the causes and treatment of autism and related neurodevelopmental disorders. He focuses primarily on nonhuman primates as models in his research and has conducted research investigations on the function of the amygdala and other parts of the primate brain.